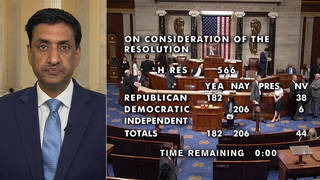
The New York Times reports the Food and Drug Administration is planning to issue stricter guidelines on any emergency use authorization of a coronavirus vaccine — even as President Trump promises a vaccine will be available as early as next month. The FDA’s emerging guidelines would affirm the warnings of researchers, who say they can’t guarantee the safety or efficacy of any of the vaccines currently in clinical trials in such a short period of time.
Topics: